It’s about 95 percent likely that you used a device with a lithium battery before you got dressed this morning. Maybe it was even more than one device: a watch, a toothbrush, a laptop or tablet, and almost certainly your smartphone. Even your car might use lithium or lithium-ion batteries. Lithium technology powers a mind-boggling amount of stuff around us—even a constantly increasing number of power tools. It’s so common and reliable that it’s easy to take it for granted. So, what is a lithium battery exactly and what are these batteries used for?
Table of Contents
- What is a Lithium Battery Made From?
- Who Invented the Lithium-ion battery?
- How Electricity Flows in a Lithium-ion Cell
- A Rechargeable Lithium Battery Reverses the Process
- What are Lithium Batteries Used For?
- Why Lithium-ion Batteries Beat Other Technology
- Types of Lithium Batteries
- What’s Wrong with Lithium-ion Batteries?
- Lithium-ion Cell vs Battery Pack
- Building a Power Tool Battery Pack
- Wrapping It Up
What is a Lithium Battery Made From?
All batteries create a current by releasing electrons through a chemical reaction. Unsurprisingly, batteries take their names from the elements involved in that reaction. Do you remember using NiMH (Nickel-Metal Hydride) batteries? Did you have tools with NiCD (Nickel-Cadmium) packs? Lithium-ion batteries use lithium, giving us some distinct advantages in the chemical reaction process that creates a current.
Who Invented the Lithium-ion battery?
We like to give credit where due, and we don’t want to leave out who invented the lithium-ion battery. That honor gets divided between three men who each contributed to what we consider a lithium-ion battery today.
Before any soft of lithium battery manufacturing took place, M. Stanley Whittingham discovered the concept of intercalation electrodes in the 1970s. That paved the way for how these batteries charged and discharged. He created the first rechargeable lithium-ion battery based on a titanium disulfide cathode and a lithium-aluminum anode.
Next, John Goodenough continued the work in 1980, using lithium cobalt oxide as a cathode. This more closely approximated a modern lithium battery and delivered a safer structure.
Lastly, the first actual modern Li-ion battery prototype came from Akira Yoshino in 1985. It used a carbonaceous anode (a material containing a large amount of carbon) instead of lithium metal. This ultimately paved the way for Sony to begin manufacturing the batteries en masse in 1991 for mobile phones.

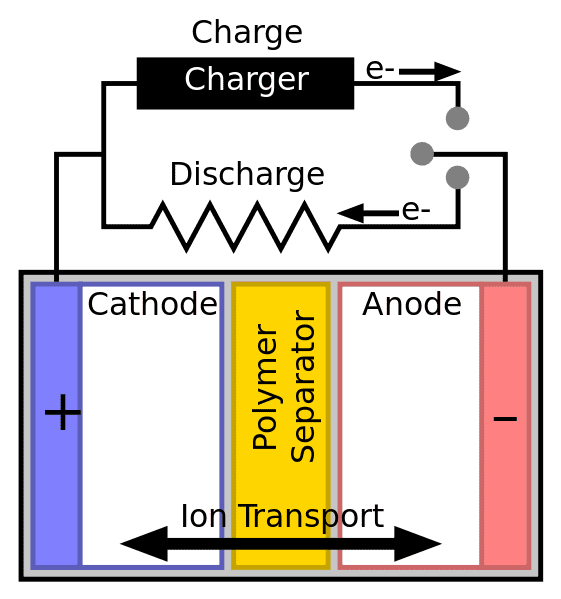
How Electricity Flows in a Lithium-ion Cell
For a battery cell to accomplish the required chemical reaction, it requires two electrodes—each with its own conductive metal. We sometimes see a cathode with aluminum and an anode with copper. The most common combination, however, features lithium cobalt oxide for the cathode and a graphite anode. We commonly find these in portable electronic devices like smartphones and laptops.
Now let’s assume the battery becomes part of a closed—or completed—circuit. That means you inserted in your cordless impact driver, for instance, and pulled the trigger.
It immediately begins discharging. An oxidation reaction occurs in the anode between the lithium and electrolyte. Electrons jump ship from the lithium atoms to create lithium ions.
The Key to Electron Flow – The Electrolyte and Separator
For this to work, you also need an electrolyte medium and a separator to keep the anode and cathode apart. The electrolyte is a sophisticated solution—Li-ion batteries typically use ether-based solutions. It will not allow electrons to pass through the microscopic holes in the separator. However, it will allow lithium ions to pass.
If you can recall your first chemistry class, you’ll remember that an ion is an atom that has a net charge, either positive or negative, due to the gain or loss of at least one electron. Electrons will follow the path of least resistance through a completed circuit, powering your impact driver in the process. The lithium ions, however, move through the separator with the help of static electricity. They meet up with their long-lost electrons in the cathode and create a reduction reaction.
Summary: A chemical reaction kicks electrons off the lithium compound and they move through the circuit. This creates energy for your tool or device. The lithium ions move through the separator to reunite on the other side.
A Rechargeable Lithium Battery Reverses the Process
Charging the battery reverses the process. Applying a current to the battery, an oxidation reaction occurs in the cathode. Then a reduction reaction occurs in the anode. Electrons move outside the cell to the opposite end. The electrolyte conducts the lithium ions through the separator again. Finally, lithium atoms again come to rest in the anode as potential energy.
We refer to non-rechargeable batteries as primary cells and rechargeable cells as secondary cells. Before secondary cells, the cathode was always the positive electrode and the anode was always the negative electrode. However, once secondary cells came along, the polarity could change.
When a secondary cell discharges, its polarity is no different than a primary cell: cathode (+), anode (-). However, after charging, a secondary cell’s cathode becomes the negative electrode, and the anode becomes the positive electrode.
Summary: Electrons and lithium ions flow one way when you use your tool or device and the opposite way when you charge the battery. Where regular batteries have defined positive and negative sides, a rechargeable battery switches depending on whether it is giving power or taking it in on a charge.
What are Lithium Batteries Used For?
Manufacturers use lithium batteries to power a wide range of products and for varied applications. These include:
- Portable consumer electronics: Products like smartphones, laptops, tablets, and other portable electronic devices take advantage of the most power density possible to reduce the volume taken up by these batteries.
- Electric vehicles (EVs): Automakers almost exclusively use lithium-ion batteries in electric vehicles and manufacture these cells in the highest quantities—driving innovation. Cells in these applications require long life, stability, and the ability to charge as quickly as possible.
- Renewable energy storage systems: These store energy from solar panels and wind turbines for use during peak demand times. It also allows for energy use when new energy creation isn’t possible (at night or when the wind dies down).
- Portable medical devices: We see lots of lithium batteries used in pacemakers, hearing aids, and other medical devices where size is key. Batteries in these applications typically feature low self-discharge rates and long lifespans.
- Aerospace: Lithium-ion batteries find ample use in spacecraft and satellites due to their high energy density and reliability.
- Military and defense: Unmanned aerial vehicles utilize lithium batteries so they can run more quietly. We also see lithium batteries in night vision devices and portable communications equipment.
While we expect lithium batteries to continue to mature—and even get replaced by new materials and technology—they certainly power a wide variety of products that would not have been possible just a few decades ago.
Why Lithium-ion Batteries Beat Other Technology
Lithium-ion batteries have won the day for several reasons. Foremost, they have a higher energy and power density pound for pound and inch for inch than their competitors. See how well a lead-acid-powered cell phone fits in your pocket! And of course, heavy batteries are really counterproductive to hybrid and fully electric cars. But like anything else, you still need to have high-quality lithium cobalt oxide (or similar) to get the best performance.
Secondly, lithium batteries are repeatedly rechargeable with minimal memory effect. That is, their maximum energy capacity doesn’t diminish over time like NiCd or NiMH batteries. They also charge faster and can hold the charge for a long time when not in use.
Types of Lithium Batteries
Within the category of lithium-ion batteries, there are several different chemistries, including lithium cobalt oxide (LCO), lithium manganese oxide (LMO), lithium nickel manganese cobalt oxide (NMC), lithium polymer batteries (LiPo), and lithium iron phosphate (LFP or LiFeP04).
NMC is one of the most popular chemistries for lithium-ion batteries because of its high energy density, long cycle life, and low cost. LFP batteries are also popular due to their high safety and long cycle life, but they have a lower energy density compared to other lithium-ion chemistries. LCO batteries have a high energy density but find less use in EVs and energy storage systems due to their difficulty handling energy-intense charging and discharging requirements.
Editor’s Note: See our article on LFP vs NMC batteries.
There are also other types of lithium batteries such as lithium titanate batteries (LTO) and Lithium Nickel Cobalt Aluminium Oxide (NCA) batteries which you can find in EV applications among others.
What’s Wrong with Lithium-ion Batteries?
Of course, it wouldn’t be real life if lithium-ion batteries didn’t have a downside. Lithium-ion batteries have several negatives. First, they often rely on rare earth elements. This makes them cost more than many other types of battery cells. Second, high temperatures can damage them. Third, you can’t fully discharge them without ruining the cell or pack—this requires special electronics and monitoring. Lastly—as you may have read—when lithium-ion cells overheat they can catch fire—and they burn hot.
And what causes that? Remember the separator—the magical boundary between electrodes that allows ions to pass but no electrons? If you damage the separator, the resulting short excites the ions rather quickly and a small fire or explosion can occur. As the song says: you gotta keep ’em separated!
For this reason, many manufacturers avoid selling individual 18650 or 2170 cells since loose lithium-ion battery cells are considered dangerous.
Summary: Lithium-ion battery cells give you more energy for the size, don’t have battery memory, charge faster, and hold their charges longer. The trade-off is higher cost and a greater risk of fire shoudl they overcharge or get punctured.
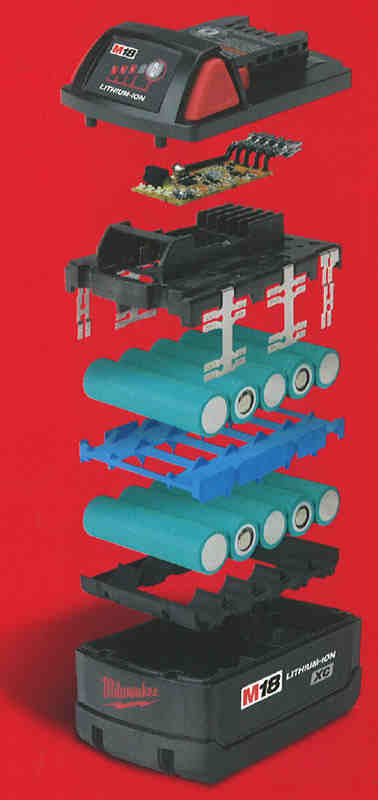
Lithium-ion Cell vs Battery Pack
Up to this point, we’ve been talking about lithium-ion cells. While some lithium-ion battery packs use just one cell, most power tool batteries use multiple cells. Engineers wire battery packs to charge or discharge the entire group of cells at the same time to provide more power and/or runtime than a single cell can do on its own.
Building a Power Tool Battery Pack
Okay, in a nutshell, what is a lithium-ion battery? It’s like any other rechargeable battery out there. It just uses lithium rather than the nickel-cadmium or nickel-metal hydride of days past. Each chemistry is different and, for now, at least, lithium is king when it comes to storing and delivering energy.
When it comes to building a lithium-ion battery pack for a power tool, manufacturers take the individual cells we’ve been describing so far and wire them with serial connections in groups of 3 (10.8V/12V), 5 (18V/20V), 6 (21.6V/24V), or 10 (36V/40V).
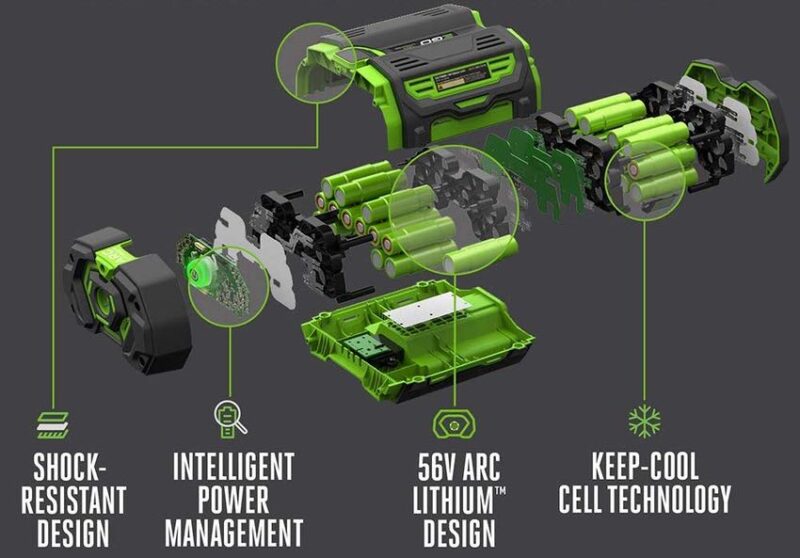
Higher voltage batteries (56V/58V/60V/80V) have even more. Usually, these are reserved for outdoor power equipment or much larger tools. The more cells you connect in serial, the higher the voltage goes.
Editor’s Note: Check out our article on 20V Max vs 18V batteries to see how these represent EXACTLY the same voltage.
Getting More Runtime or Power
When you take groups of cells, like 2 groups of 5, and use a parallel connection, you increase the amp hours. This effectively gives you the option to run tools longer or with greater amounts of power at the same voltage. Check out our article about voltage vs amp hours for more details on how these capacities relate to one another.

Taking those groups of cells, manufacturers then encase them. This helps dissipate heat and protect the cells from dirty and sometimes wet jobsite conditions. They sometimes add electronic chips to protect it during charging and use. Viola! You have a power tool battery pack.
Summary: A power tool battery takes individual lithium-ion cells and puts them together in a pack that also helps cool the battery and monitor its state electronically.
Wrapping It Up
It all sounds like a little chemistry magic à la Walter White’s fulminated Mercury. But it’s really happening all around us constantly. It helps us stay connected and productive…and foment social unrest online—or at least argue with people we used to like a lot more when we knew them only in real life!
We hope that you’re positively charged to be here today. If you see something we missed or have any questions—be sure to leave them in the comments below.